Cancer killers send signal of success
New cancer-fighting nanoparticles deliver results — and status reports.
Tiny biochemical bundles carry chemotherapy drugs into tumors and light up when surrounding cancer cells start dying. Future iterations of these lab-made particles could allow doctors to monitor the effects of cancer treatment in real time, researchers report the week of March 28 in the Proceedings of the National Academy of Sciences.
“This is the first system that allows you to read out whether your drug is working or not,” says study coauthor Shiladitya Sengupta, a bioengineer at Brigham and Women’s Hospital in Boston.
Each roughly 100-nanometer-wide particle consists of a drug and a fluorescent dye linked to a coiled molecular chain. Before the particles enter cells, the dye is tethered to a “quencher” molecule that prevents it from lighting up. When injected into the bloodstream of a mouse with cancer, the nanoparticles accumulate in tumor cells and release the drug, which activates a protein that tears a cancer cell apart. This cell-splitting protein not only kills the tumor cell, but also severs the link between the dye and the quencher, allowing the nanoparticles to glow under infrared light.
Previous techniques could track drugs entering tumors, but that “doesn’t necessarily tell you whether the drug is working or not,” says study coauthor Ashish Kulkarni, a bioengineer at Brigham and Women’s and at Harvard Medical School.
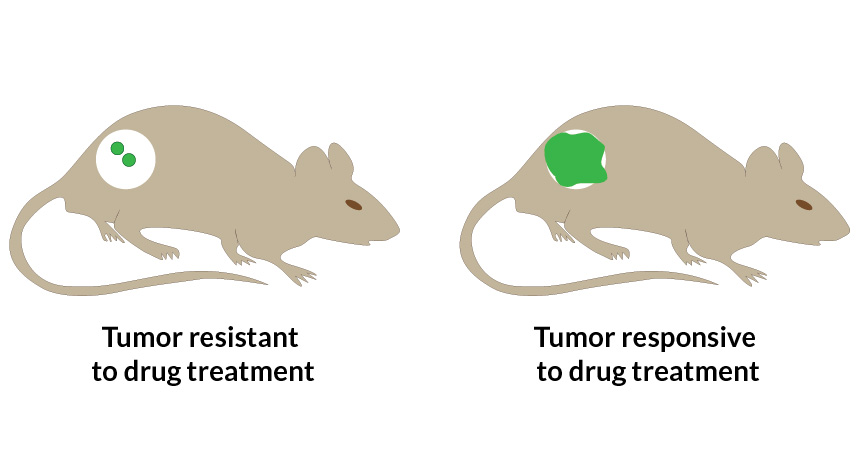
The team tested the nanoparticles in mice that each had two types of tumor: one resistant to the drug in the particles and one responsive to the drug. Drug-sensitive tumors glowed around five times as intensely as the resistant tumors. Results were swift, with tumors lighting up in eight to 12 hours.
Replacing the particles’ cancer drug with antibodies that summoned the body’s tumor-fighting defenses allowed the team to test the nanoparticles as immunotherapy agents. In this case, tumors lit up after five days, reflecting an initial lag time of immunotherapy compared with chemotherapy.
These nanoparticles are a proof of concept, Sengupta says. Next steps include redesigning the nanoparticles using clinically approved materials and dyes that would be easier to track in the human body with the use of an MRI machine. But such imaging chemicals can be toxic, which could pose a problem for the nanoparticle design, says cancer nanotechnologist Mansoor Amiji of Northeastern University in Boston. Dyes should be cleared from the body as quickly as possible, while the drug they’re paired with might take weeks to work. But the study’s focus on detecting drug performance in real time is very important, and demands further study, Amiji says. “There’s tremendous need, especially as we think about personalizing cancer therapies.”